Paving the Way for Rare Disease Gene Therapies
Platform Vector Gene Therapy (PaVe-GT) – a National Institutes of Health (NIH) initiative to increase the efficiency of AAV-based gene therapy development for rare diseases
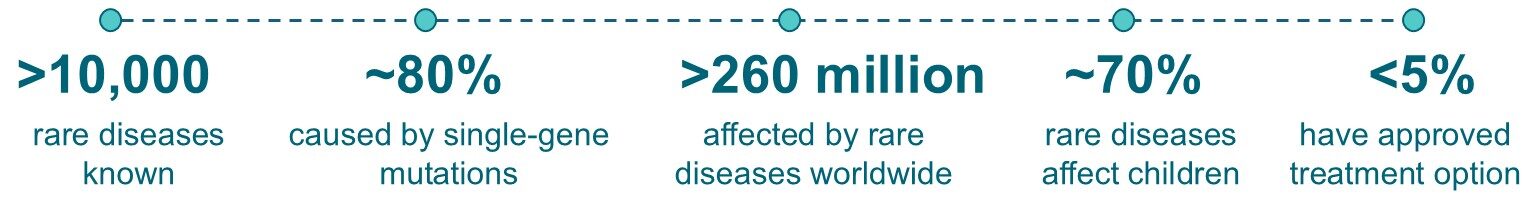
With more than 10,000 bona fide rare diseases reported in the biomedical literature collectively, rare diseases are not rare. Of these, more than 80% are caused by mutations in single genes and are therefore amenable to gene therapy. Currently, more than 95% of rare diseases do not have approved therapy. 1-5

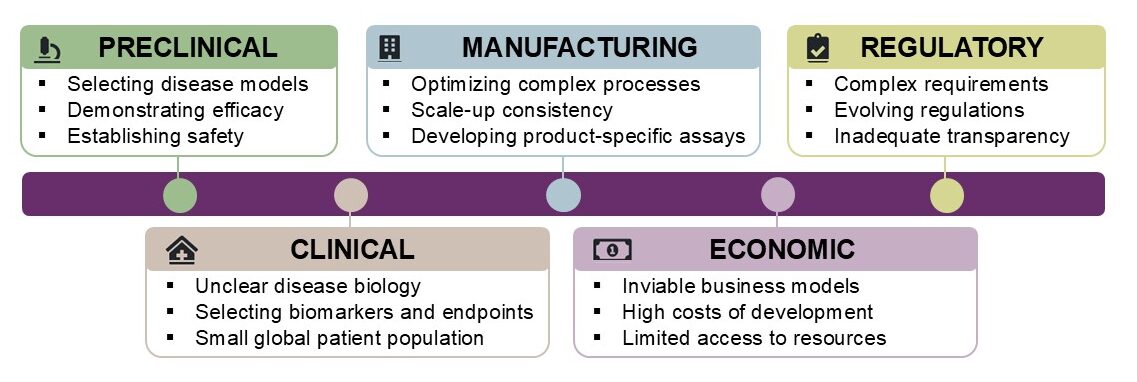
Therapies that replace or fix defective genes hold the potential of long-term benefit for patients with rare diseases caused by mutations in a single gene (monogenic diseases). However, the development of gene therapies continues to face many scientific, clinical, regulatory, and economic challenges.
Hypothesis: A platform vector approach to gene therapies with AAV increases efficiencies in preclinical testing and clinical trial startup for related rare diseases.6-7
Goals: PaVe-GT aims to accelerate the development of adeno-associated virus (AAV)-based gene therapies for rare genetic diseases by:
Approach: Use AAV vectors as a gene delivery platform with similar protocols and processes for more efficient therapeutic translation.
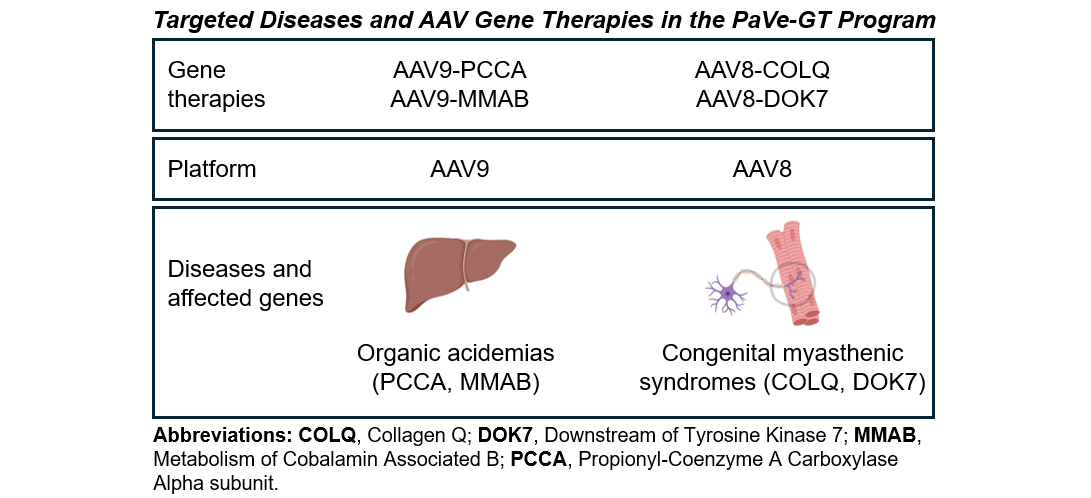
Advantage: The use of AAV as a gene delivery system may allow leveraging of information across the platform and enable efficient development of other AAV gene therapies.
PaVe-GT is a collaborative effort that applies National Center for Advancing Translational Sciences’ (NCATS’) translational science model by bringing experts from multiple partners together to work as a single team. The multidisciplinary PaVe-GT team consists of basic researchers, disease and drug development experts across NCATS, the National Human Genome Research Institute (NHGRI), National Institute of Neurological Disorders and Stroke (NINDS), NIH Clinical Center (CC), and the National Institute of Child Health and Human Development (NICHD).
Receive the latest updates from the PaVe-GT team and press releases.
The PaVe-GT team has gained widely applicable first-hand experience in gene therapy development and is publicly sharing translatable scientific and regulatory knowledge to promote the development of other gene therapies. Utilize these PaVe-GT regulatory packages, target product profiles, templates and more, as examples to inform your gene therapy program.
Resources that will be shared over the course of the PaVe-GT program include: