Frequently Asked Questions
In addition to the information below, please visit our Resources page for patient-friendly explanations of gene therapy.
Vectors deliver genes to cells and tissues in the body where the gene can have a treatment effect. AAV, or adeno-associated virus, was identified by laboratory scientists many years ago. PaVe-GT is developing four AAV-vector mediated gene therapies. AAV does not cause disease in humans. AAV is the vector for the four PaVe-GT gene therapies. Different types of AAV (sometimes called “strains” or “serotypes”) target different cells and organs within the body. PaVe-GT will use AAV9 to create gene therapies because it can deliver to the liver, brain, heart, and other target cells and organs of interest for the diseases being studied.
Viruses are composed of an outer protein coating, known as a capsid, and genetic material (such as DNA) within the capsid. See Figure 1.

In AAV-mediated gene therapy, most of the genes in the AAV particle are removed and replaced with the human gene that is being used as the therapy. See Figure 2.
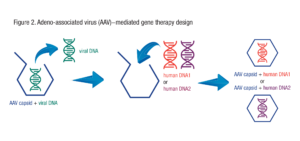
By putting different therapeutic genes within the AAV capsid, different AAV-mediated gene therapies can be produced. This is why AAV is sometimes called a “platform” therapeutic. It is a platform to deliver genes.
As an illustrative example, you can think of AAV as a shipping box.
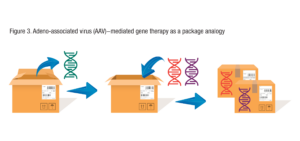
In AAV-mediated gene therapy, the AAV capsid is acting like a shipping box, carrying the gene of interest to the right destination. As long as the therapeutic gene fits in the shipping box, and the box is packed and sealed appropriately, and the address on the box is part of the mail system’s list of serviceable addresses, the mail system will get the contents to its proper destination. The content of the box is revealed and relevant only when the box has been delivered. In theory, AAV-mediated therapy works the same way: Different genes can be inserted into the AAV capsid and the body will be seeing the same thing every time – the AAV capsid – that will carry and target the gene for delivery to the right destination. That is why AAV and other platform gene therapy vectors could be useful for treating many diverse genetic diseases.
PaVe-GT stands for Platform Vector Gene Therapy. PaVe-GT is a pilot project that will develop gene therapies for four different diseases in a streamlined way.
The main goal of PaVe-GT is to test whether we can significantly increase the efficiency of gene therapy clinical trial startup by using a standardized process with the same capsid and manufacturing methods for all four diseases under study.
Another goal is to share project results and lessons learned with the public in such a way that the information is useful to any party interested in developing a gene therapy efficiently. Specifically, we will make information and results from the PaVe-GT program publicly available in as timely a manner as possible. This includes toxicology and biodistribution data, Investigational New Drug filings and communications with the U.S. Food and Drug Administration, and other study documents.
An estimated 6,500 inherited (genetic) diseases are caused by a defect in a single gene. In theory, many of these diseases could be treated with a gene therapy using adeno-associated virus (AAV) vectors. Scientists across the world have conducted proof-of-concept studies for AAV gene therapies for many rare diseases. Yet there are only a small number of clinical trials, many of which focus on a small number of diseases, for which there are several thousand patients identified. Due to the expense and consolidation of expertise, most of these studies are sponsored by pharmaceutical and biotech companies (“industry”).
The potential to develop gene therapies for many more diseases is increasingly becoming possible. For many rare diseases, the limiting factor is not scientific knowledge, but rather operational and financial hurdles, particularly for diseases that have very small numbers of patients (a few hundred or fewer people in the United States). PaVe-GT will be a public model of how to plan and conduct a clinical trial for gene therapies for very small groups of patients, which are unlikely to ever be of commercial interest. NCATS is also working with other groups in efforts to reduce the cost of AAV vector manufacturing and to address other common problems in gene therapy development.
Creating a new gene therapy is a very long, expensive and complicated process. PaVe-GT might “pave the way” and serve as a model for future projects, but there will still be important and resource-intensive, disease-specific work for each follow-on gene therapy, such as the creation of relevant animal models for use in testing efficacy, design and testing of the therapeutic gene, manufacturing of clinical-grade therapeutic material, and clinical trial costs. PaVe-GT is one of several NCATS programs to streamline the development of gene therapies for rare diseases.
Importantly, the focus of the PaVe-GT program is not on the specific diseases, but rather on increasing the efficiency of starting a gene therapy clinical trial. PaVe-GT will develop gene therapies for four diseases: two congenital myasthenic syndromes and two organic acidemias. The congenital myasthenic syndromes under study are Dok7 deficiency and ColQ deficiency. The organic acidemias under study are propionic acidemia (caused by PCCA deficiency) and isolated methylmalonic acidemia (MMAB deficiency/cobalamin type B methylmalonic acidemia).
There are many unknowns in pilot projects. They carry a high risk of failure and are likely to encounter unexpected research problems that will need to be solved. In order to increase the likelihood that the PaVe-GT approach will be successful, NIH has decided to conduct PaVe-GT using NIH investigators and facilities. This decision allows us to carry out all four trials in the same institution and to leverage the unique resources of the NIH Clinical Center. In addition, since all personnel involved work for the federal government, we can avoid concerns about the protection of intellectual property that could arise if the trial was carried out in a university. This is of paramount importance, given that a major goal of PaVe-GT is to put project results and documents into the public domain so that they can be made freely available to others.
These diseases were identified as suitable for PaVe-GT for the following reasons:
- They are under study by investigators at the NIH Clinical Center.
- They are serious diseases with no effective treatments or therapies available, such that the inherent risks of gene therapy are balanced by the potential clinical benefit.
- They are amenable to AAV9-mediated gene therapy. In each case, the gene is of small enough size to fit into the AAV9 vector, and cells, tissues and organs to which the gene will be delivered can be targeted by AAV9.
- The diseases are well understood at a molecular and biochemical level.
- Their natural history is understood, and outcome measures have been identified.
- The NIH clinical investigators have years of experience with these diseases.
- Sufficient numbers of patients are available for the clinical trials
- No ongoing gene therapy development for any of the diseases could be identified at the beginning of the project, and given the small patient population with the diseases, future commercial interest in the disease was thought to be unlikely.
The pilot diseases were carefully selected, and we do not have the capacity to add more diseases to this pilot project at the present time. However, the results from PaVe-GT are intended to benefit many different gene therapy clinical trials in the future, and information developed in PaVe-GT will be shared publicly on the NIH PaVe-GT website so that everyone can benefit from it.
NCATS has arranged intra-NIH partnerships with other NIH Institutes and Centers, including the National Institute of Neurological Disorders and Stroke (NINDS); the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD); the National Human Genome Research Institute (NHGRI); and the NIH research hospital, the Clinical Center (CC).
The lead investigators for the clinical research are:
- Carsten Bönnemann, M.D., Senior Investigator, NINDS
- Charles Venditti, M.D., Ph.D., Senior Investigator, NHGRI
The lead NCATS Offices and Divisions include the Office of Rare Diseases Research (ORDR), the Division of Preclinical Innovation (DPI), the Therapeutics for Rare and Neglected Diseases (TRND) program within DPI, and the Office of Strategic Alliances (OSA).
This pdf file contains the RPD designation request for AAV9-hPCCA (NCATSBL-0746) and associated communications between NCATS and FDA OOPD.
This pdf file contains the ODD request for AAV9-hPCCA (NCATSBL-0746) and associated communications between NCATS and FDA OOPD.






